We all know that hydrocarbons are the vital and most prominent compounds of organic chemistry. From the definition of organic chemistry, we get to know that it is the branch of chemistry that deals with the scientific study of organic compounds; these are the compounds that contain covalently bonded carbon atoms. Organic chemistry and hydrocarbon compounds go hand in hand. Hydrocarbons are the fundamental compounds in organic chemistry. A wide variety of compounds with different classes, structures and groups come under the shelter of hydrocarbons. In this article, we will throw some light on the few important features and highlights of these hydrocarbons.
What are Hydrocarbons?
As the name suggests, hydrocarbons are organic compounds that are completely made up of only two sorts of atoms that is carbon and hydrogen. In general, hydrocarbons are colourless gases that have very delicate odours. These molecules can possess simple or relatively complex structures. The structure of hydrocarbons helps in determining their properties, shape and to which classification they belong.
Types
There are many classes and types of hydrocarbons. A wide range of compounds with different structures such as terpenes, anhydrides and many more are classified on these types.
Listed below are the types of hydrocarbons:
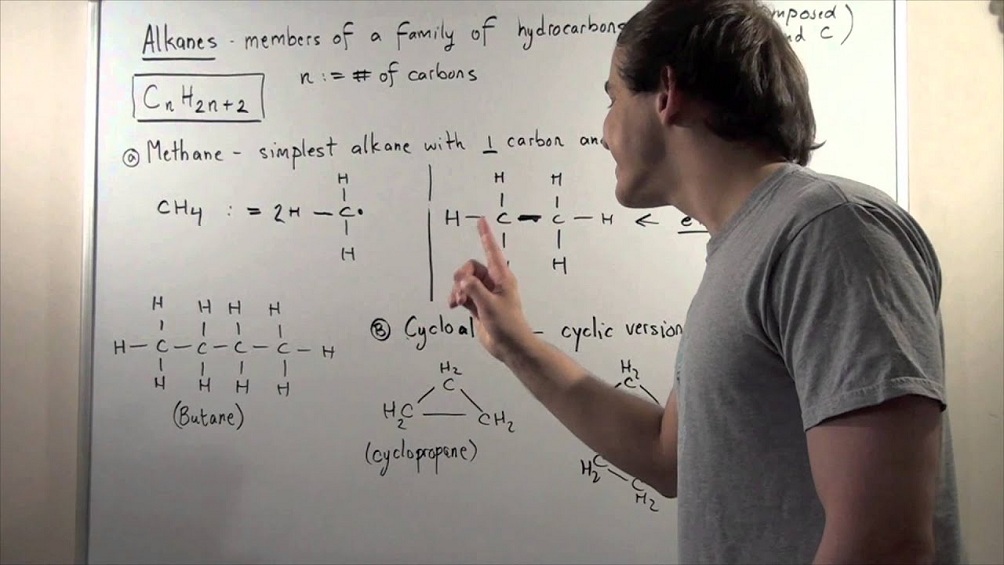
- Saturated Hydrocarbons: In these types of compounds, single bonds are present between the carbon-carbon atoms. They are together called alkanes which have a general formula CnH2n+2
- Unsaturated Hydrocarbons: In these types of compounds, double or triple bond between carbon-carbon atoms. The double-bonded compounds are known as alkenes, and the triple bonded compounds are known as alkynes. The general formula for alkenes is CnH2n, and similarly, for alkynes, the general formula is CnH2n-2.
- Cycloalkanes: These hydrocarbons consist of one or multiple carbon rings. The hydrogen atom present is attached to the carbon ring.
- Aromatic Hydrocarbons: These are also called arenes. Compounds that consist of at least one aromatic ring are known as arenes.
- Aliphatic Hydrocarbons: The straight-chain structures that do not contain any ring in them are called aliphatic hydrocarbons.
- Alicyclic Hydrocarbons: The hydrocarbons that possess a ring structure in them are known as alicyclic hydrocarbons.
Reactions of Hydrocarbons
Each and every hydrocarbon is different from another hydrocarbon. The structure, atoms present in it and also its chemical properties define how each one reacts with another substance.
- Oxidation, combustion, aromatisation, and free radical substitution are the major reactions that are undergone by alkanes.
- An addition reaction is mainly an electrophilic addition reaction that is undergone by unsaturated compounds such as alkenes and alkynes.
Aromatic hydrocarbons are mainly involved in electrophilic substitution reactions. Some other important chemical reactions such as ester formation reaction, saponification, acylation are examples of reactions that possess these hydrocarbon molecules.
Availability and Preparation
These compounds are available in nature as well as can be prepared synthetically in laboratories. Listed below are the points on availability and preparation.
- Hydrocarbons occur naturally in plants and animals. It is also found in fossils that have been created by various factors such as temperature, pressure and weight over millennia. They are mostly extracted from deep underground, in porous rock formations.
- In the laboratory, the hydrocarbons can be prepared by various reactions using the Sabatier-Sender son’s reaction. The various catalysts involved in the preparation of hydrocarbons are Pt, Pd-BaSo4, Adams catalyst (Pt2O) or Wilkinson catalyst (R3PRhCl), etc. Most of the reactions that involve the preparation of alkenes undergo the elimination process. For instance, alkynes can be prepared from alkyl halides and alcohols.
Applications
This class of molecules with a wide variety of compounds have spread out its applications in various fields. Listed below are the few uses of hydrocarbons:
- Hydrocarbons are prominently used as fuels. LPG (liquefied petroleum gas) and CNG (liquefied natural gas) are vital examples of hydrocarbon fuels.
- They are used in the manufacturing of polymers such as polyethene, polystyrene etc.
- These are also utilised in the manufacturing of drugs and dyes as a base material.
- Lubricating oil and grease are also the products of hydrocarbons.
This class of compounds have numerous compounds and can be found everywhere. Hydrocarbons are the vital group of compounds in organic chemistry and help in manufacturing a small product such as soap to the vital compound fuel. These play an important role in medicinal chemistry, industrial applications and also in the production of compounds in small scale industries.